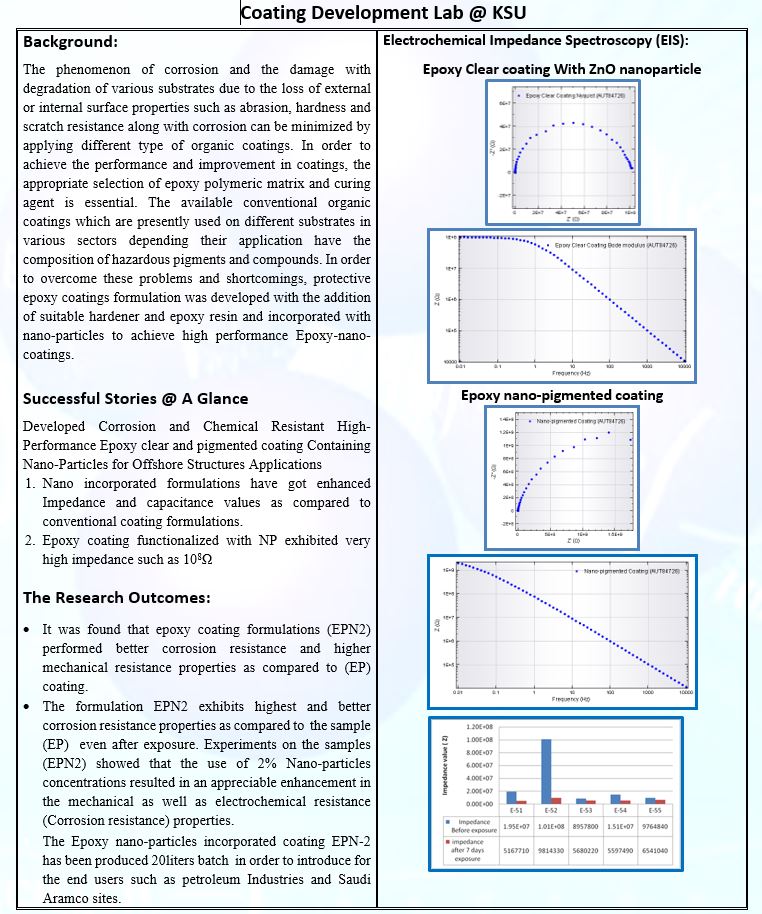
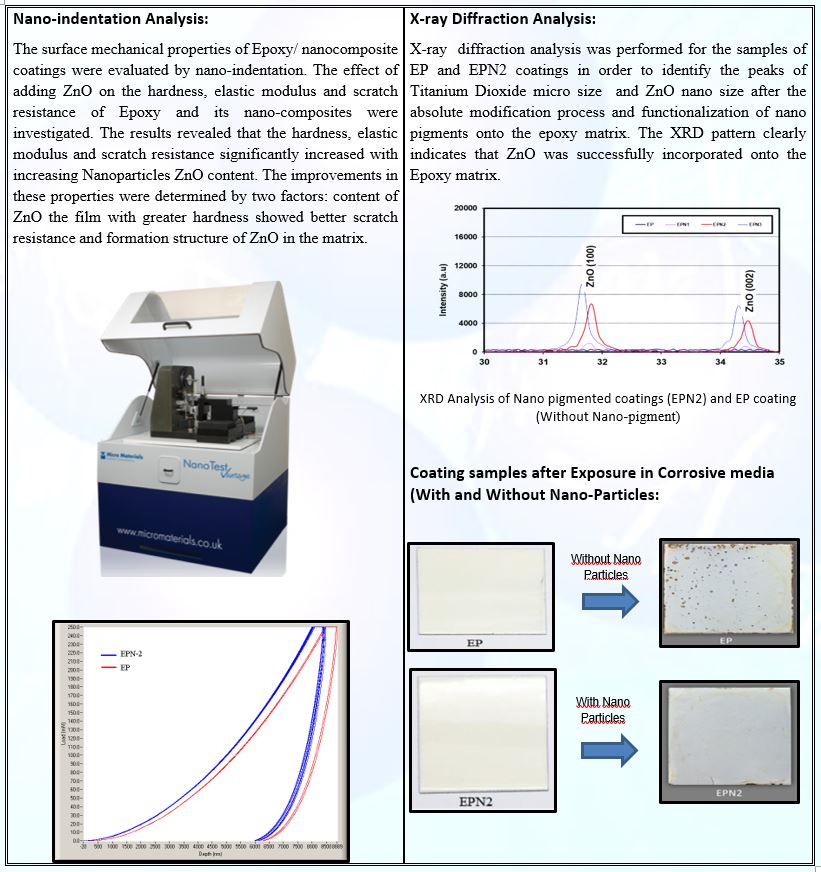
Research Focus
A. Metallic Materials
CEREM research is focused on the development of nanostructure materials, high strength low alloy steels and super alloys as well as texture analysis of metallic materials with advanced characterization techniques such as XRD, EBSD, EDX, SEM and TEM etc. Many other research topics were also focused such as Metal Matrix Composites, Polymers and Polymers Composites, Metallurgy, Tissue Engineering, Tribology, High Frequency Induction Heat Sintering, Electrochemical Corrosion Studies, Electrospinning of nanofibers.
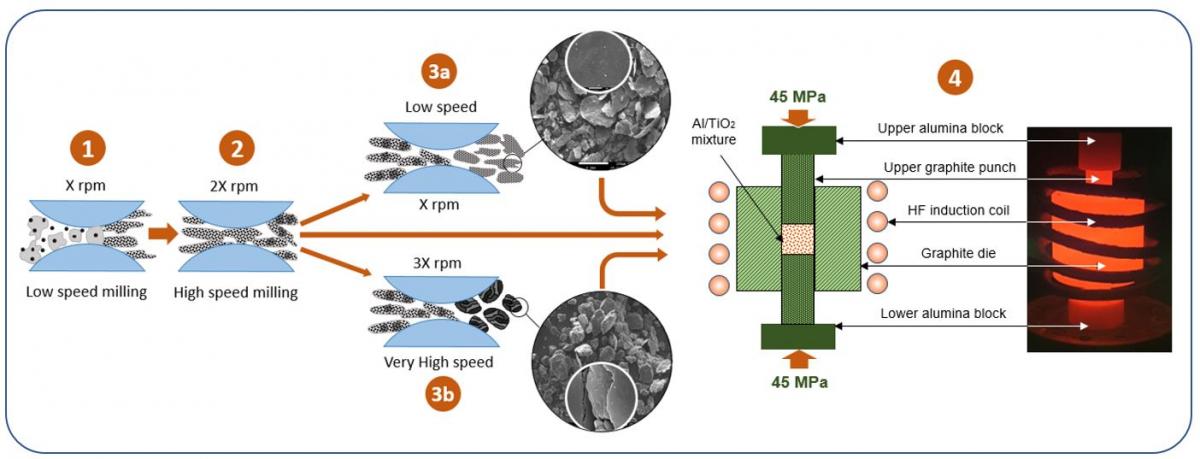
Ball milling process through powder metallurgy route: for mixing and particle size reduction.
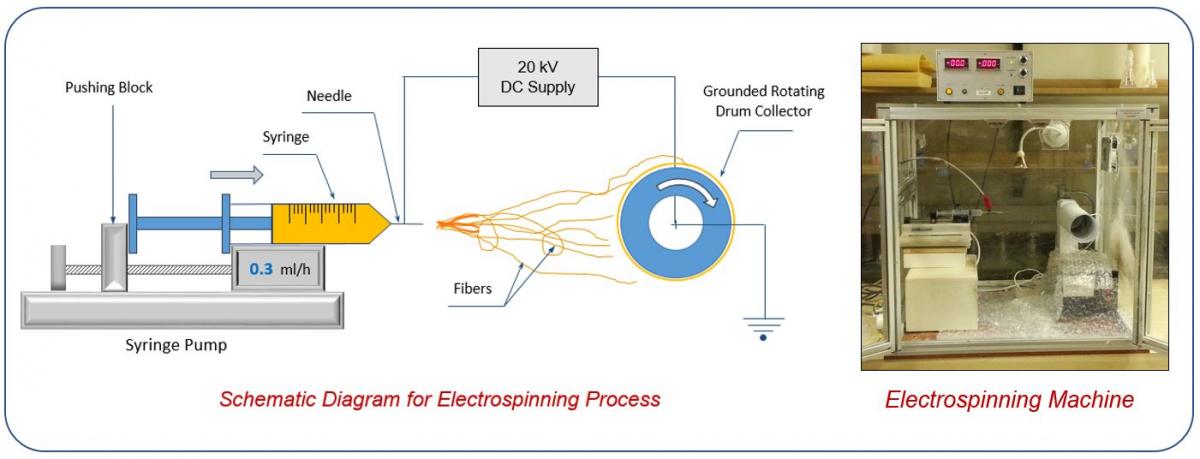


B. Composites Materials
Revolutionary advances in composites occurred from the use of Nanotechnology in self-healing material development and other high performance materials. The Center is focused on the technological advancements in Nanocomposite and will provide an efficient and timely transfer and implementation of new technology and techniques to the industry.
1. Energy storage.
Graphene, a two dimensional (2D) layered material has attracted much attention from the scientific community due to its exceptional electrical, thermal, mechanical, biomedical and optical properties. Hence, numerous applications utilizing graphene-based materials could be conceived in the next generation electronics, chemical and biological sensing, energy conversion and storage, and beyond. Interaction between graphene surfaces with other materials, plays a vital role in influencing its properties than other bulk materials.
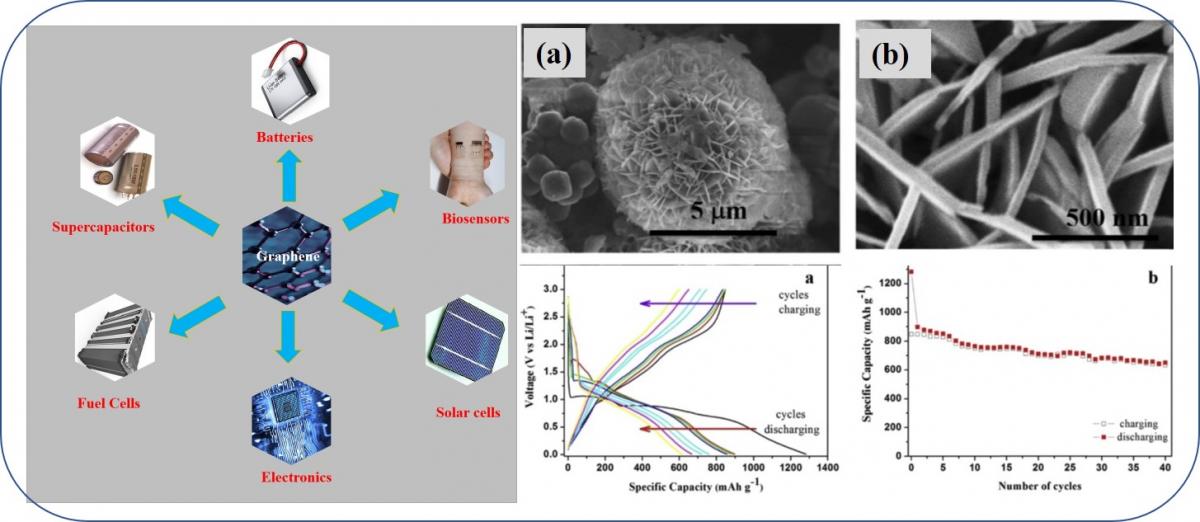
Graphene Based Nanostructures composite materials for Energy Storage, Energy Conversion and Biomedical Applications.
2. Electrochemical sensor and biosensors.
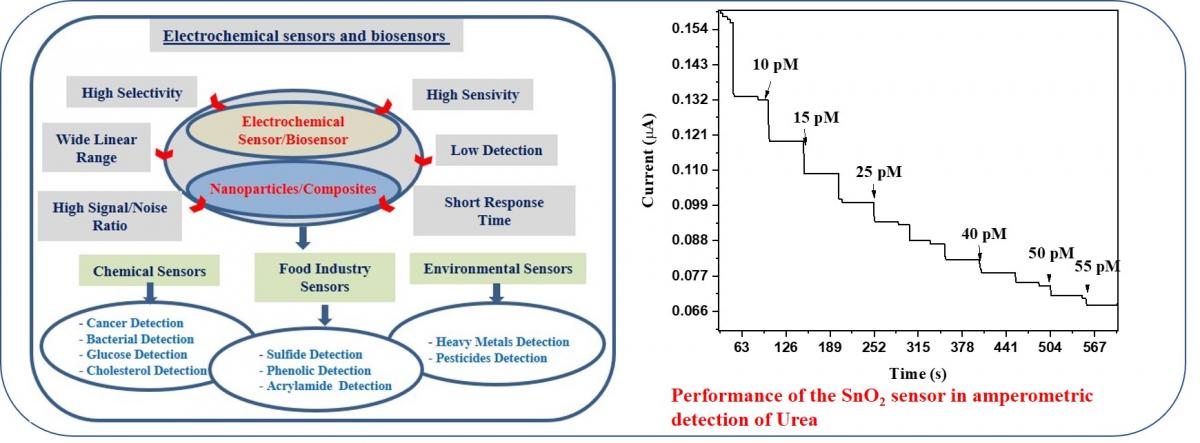
3. Nanomaterials/Polymers/composites.
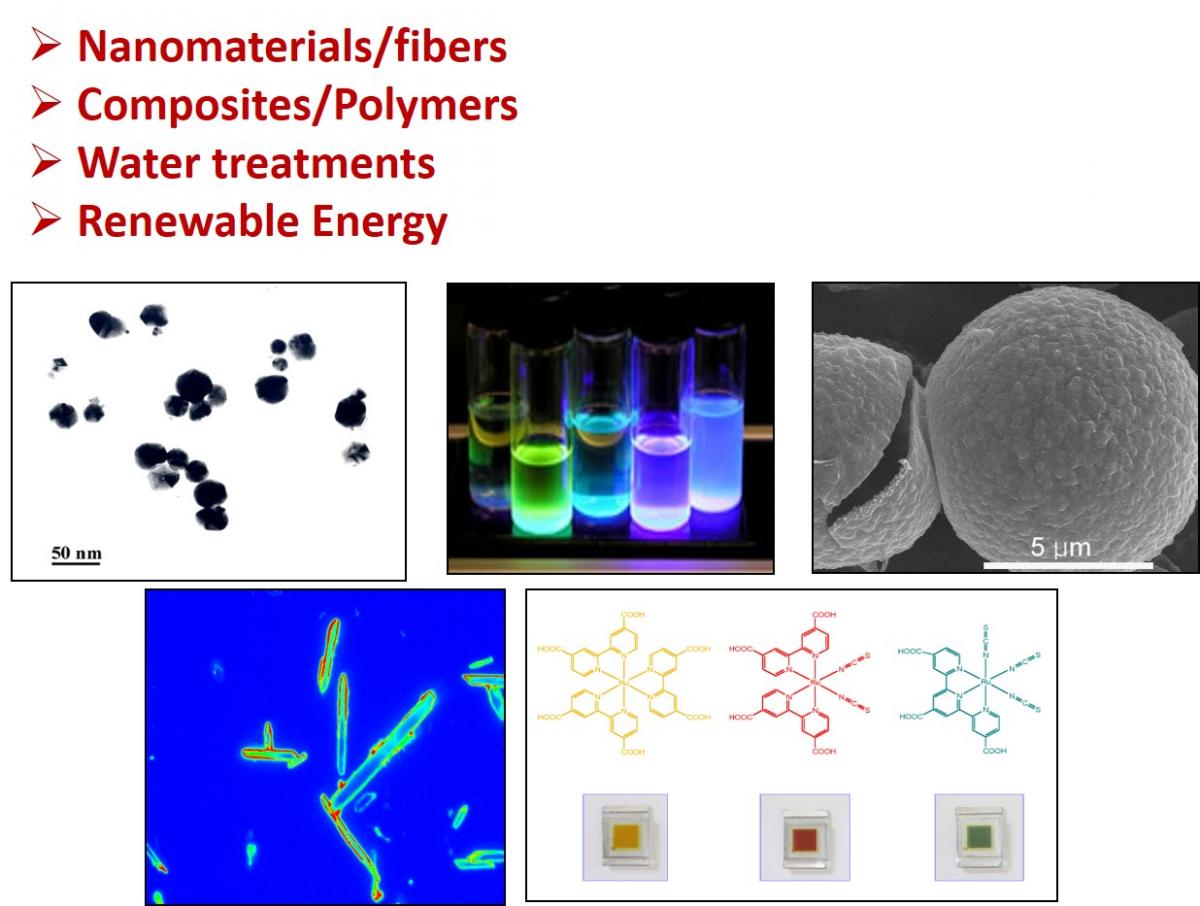
C. Corrosion
Due to the harsh environment and aggressive nature of the industries, Corrosion investigations is a major area of interest for CEREM that includes corrosion of plant components, rotating equipments, pipelines, storage tanks, bridges and platforms. The Center acts as the leading source of information on the different techniques for corrosion protection and encourages research to ensure better understanding of the preservation of the structures under severe environmental conditions.
We have been working on the fabrication of new alloys and studying their mechanical properties in simulated body fluid. The work also is extended to involve the synthesis of new corrosion inhibitors and reporting their inhibition efficiency on the corrosion of metals in a synthetic seawater medium.
1. Development and Characterization of Ti- Zr-Ta-Sn Alloys for Biomedical Applications
In this study, the fabrication of pure Ti and Ti-Zr, Ti-Zr-Ta, and Ti-Zr-Ta-Sn alloys has been performed. The mechanical properties as well as the corrosion resistance of these alloys in simulated body fluid have been carried out.
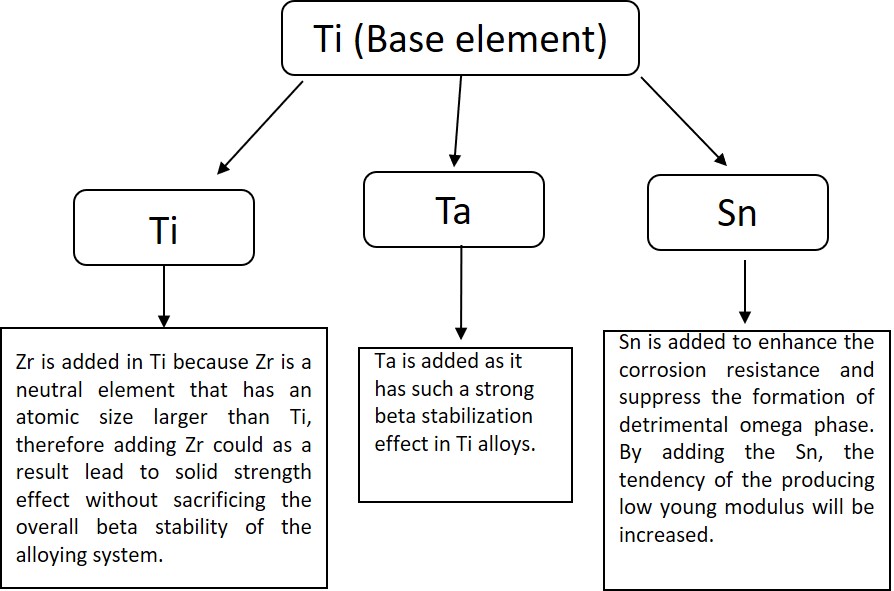
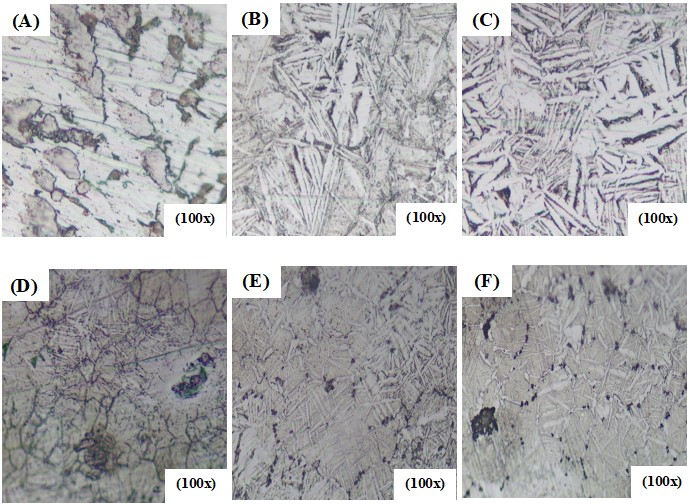
Figure 1. Optical microstructure of (a) Ti, (b) Ti-15%Zr, (c) Ti-15%Zr-2%Ta, (d) Ti-15%Zr-2%Ta-4Sn% (e)Ti-15%Zr-2%Ta-6Sn% and (f) Ta Ti-15%Zr-2% Ta – 8%Sn.
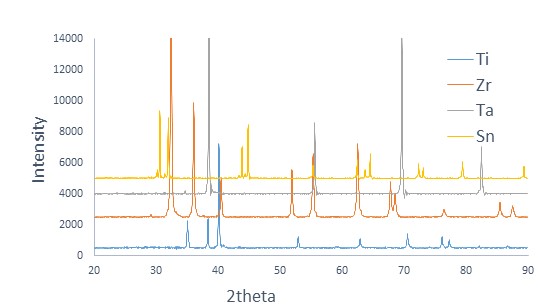
Figure2. XRD patterns of the as-received elemental powders
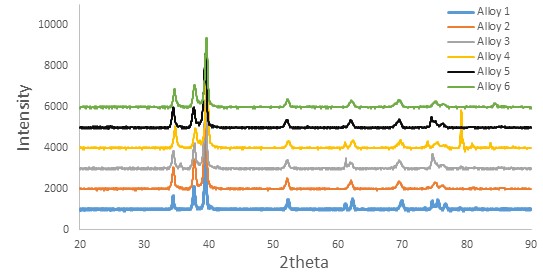
Figure 3:XRD patterns of sintered alloys, alloy1(Ti), alloy 2 (Ti-15zr), alloy 3 (Ti-15Zr – 2Ta), alloy 4 (Ti-15Zr-2Ta-4Sn), alloy 5 (alloy2 Ti-15Zr-2Ta-Sn6) and alloy 6 (Ti-15Zr-2Ta -8 Sn).
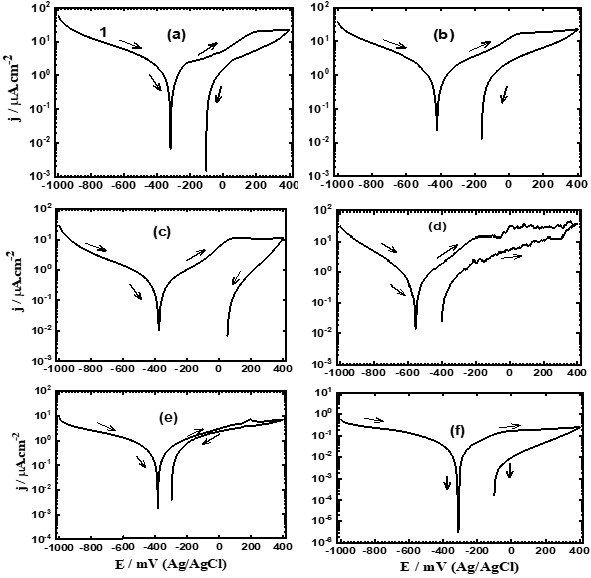
Figure 4. CPP curves of (a) Ti, (b) Ti-15%Zr, (c) Ti-15%Zr-2%Ta, (d) Ti-15%Zr-2%Ta-4Sn% (e)Ti-15%Zr-2%Ta-6Sn% and (f) Ta Ti-15%Zr-2% Ta – 8%Sn after 1h immersion in simulated body fluids.
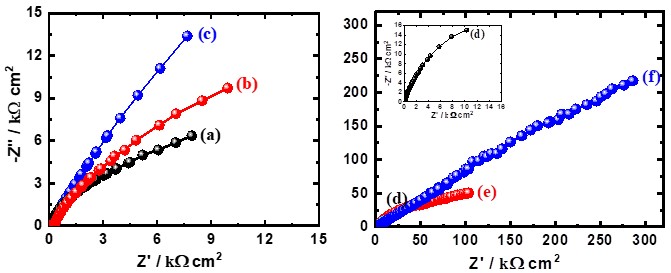
Figure 5. Nyquist plots measured (symbols) and calculated (lines) for (a) Ti, (b) Ti-15%Zr, (c) Ti-15%Zr-2%Ta, (d) Ti-15%Zr-2%Ta-4Sn% (e)Ti-15%Zr-2%Ta-6Sn% and (f) Ta Ti-15%Zr-2% Ta - 8Sn% after 1h immersion in simulated body fluids.
2. Inhibition of metals and alloys in harsh environments using synthetic corrosion inhibitors
In this kind of work, the alleviation of iron corrosion in 3.5% NaCl sodium chloride solution using the new synthesized N,N'-bis[2-methoxynaphthylidene]amino]oxamide (MAO) as a corrosion inhibitor has been reported. The work was achieved using various techniques such as potentiodynamic cyclic polarization (PCP) displayed a powerful inhibition for the corrosion via reducing the iron’s anodic and anodic reactions.
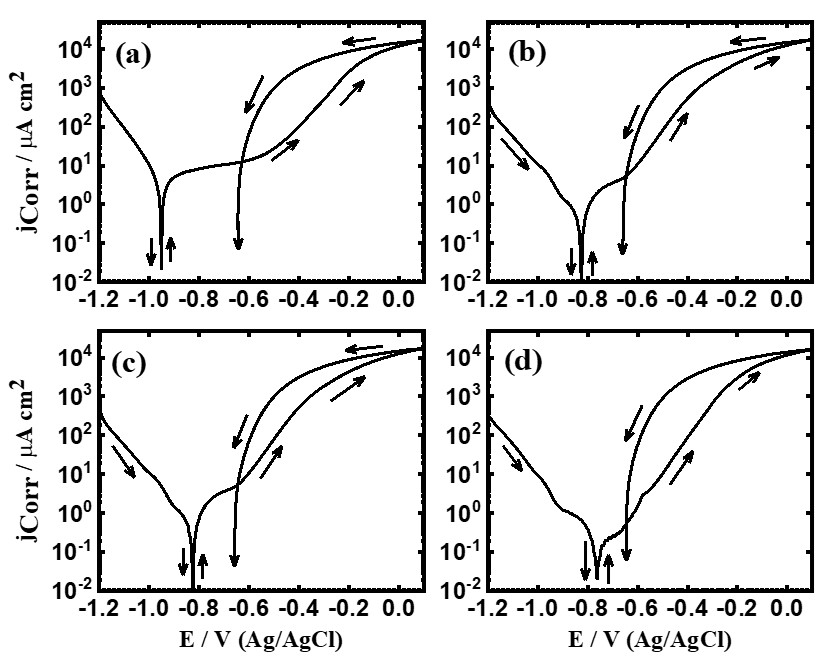
Figure 1. PCP curves for iron rod after 1 h immersion in (a) 3.5% NaCl without and with (b) 5x10-5 M MAO, (c) 1x10-4 M MAO, and (d) 5x10-5 M MAO present, respectively.
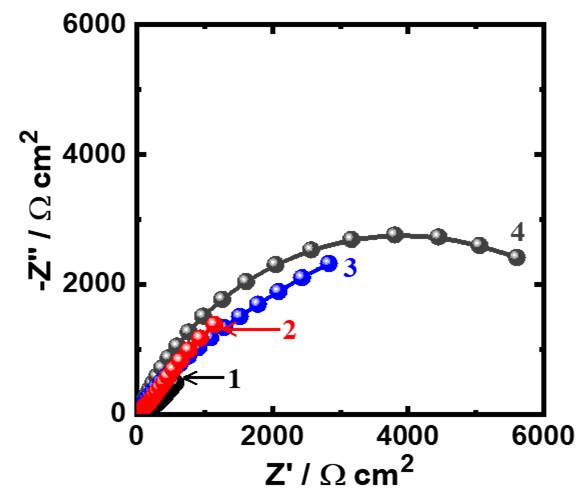
Figure 2. Nyquist spectra for iron after its exposure for 1 h to (1) 3.5% NaCl solutions in absence and presence of (2) 5x10-5 M MAO, (3) 1x10-4 M MAO, and (4) 5x10-5 M MAO, respectively.
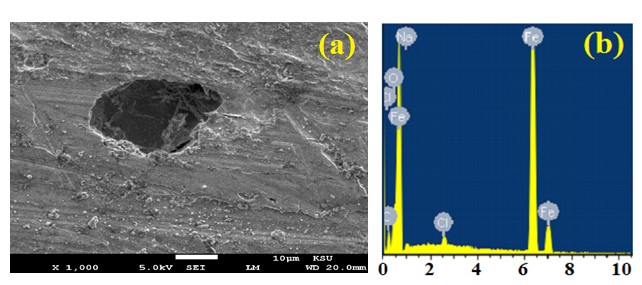
Figure 3. SEM image and EDX profile spectrum after 1 h holding the potential at -475 mV (Ag/AgCl) for the surface of the iron rod that was immersed for 48 h in 3.5% NaCl solution.
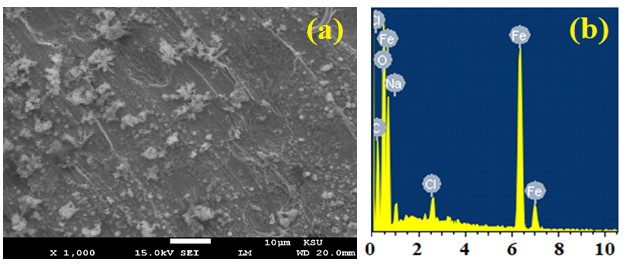
Figure 7. SEM image and EDX profile spectrum after 1 h holding the potential at -475 mV (Ag/AgCl) for the surface of the iron rod that was immersed for 48 h in 3.5% NaCl + 5x10-4 M MAO solution.
D. Coatings: